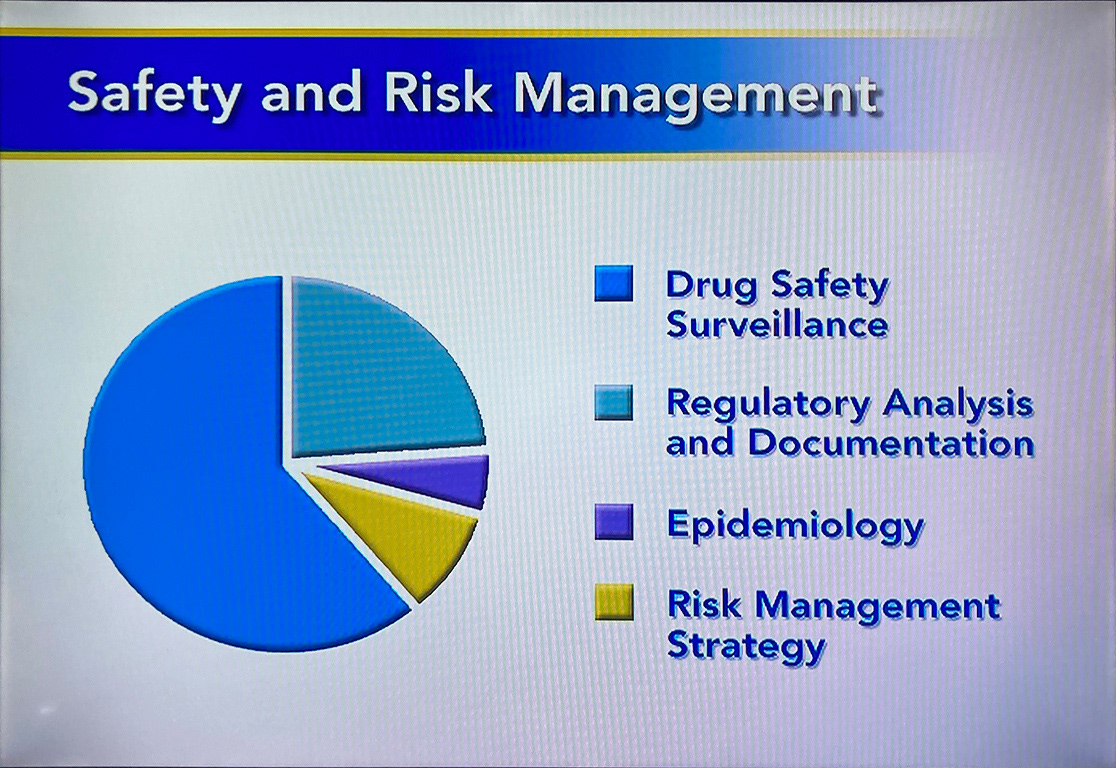
Safety and Risk Management
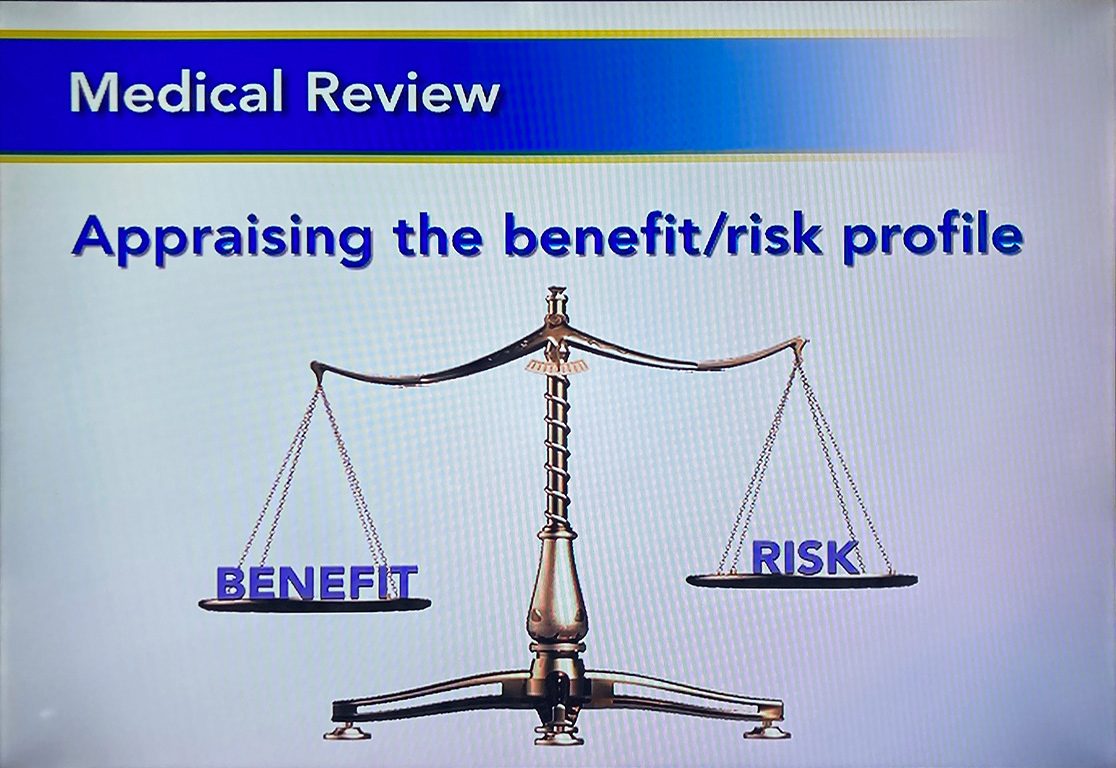
The pharmacovigilance group at Pfizer is responsible for tracking drug safety and efficacy as well as investigating reports of adverse reactions during clinical trials and after FDA approval.
Pfizer was about to introduce a new software system, Argus, to its employees in the Safety and Risk Management group. Before the system was to go "live," an extensive training program was put in place and promoted through a series of workplace posters.
Pharmacovigilance at Pfizer (DVD Video)
Graphics and chyrons created for a short video presentation that explained the drug safety and risk management review processes at Pfizer.








Pfizer Presentation Materials
A portion of a Pfizer presentation for the drugs Cabaser and Dostinex. This timeline was presented as both a standalone poster and as part of a PowerPoint presentation for a conference.
Another timeline for the Pfizer drug Aricept, presented as two posters for a conference.